- Adani Hindenburg Case: SEBI Clears Adani Group of Hindenburg Allegations.
- The 10-Minute Rule by Steve Jobs - A Stanford-Proven Creativity Hack
- Hillhouse buys minority stake in Carlyle-backed Quest Global at $4.5b valuation
- ‘Jailed a tantri for revenge’: BJP, Congress launch fresh offensive against Pinarayi Vijayan govt over Sabarimala
- Antimicrobial Textile Market Size to Reach USD 25.55 Billion by 2035
Recent 2025 research, including a 766-patient study presented at ESCRS, reveals that eye drops containing pilocarpine and diclofenac can significantly improve near vision for those with presbyopia, the age-related loss of close-up focus. These drops offer a non-invasive solution, reducing reliance on glasses with sustained results for up to two years and minimal side effects.
Eye Drops That Could Eliminate Reading Glasses: Research Breakdown
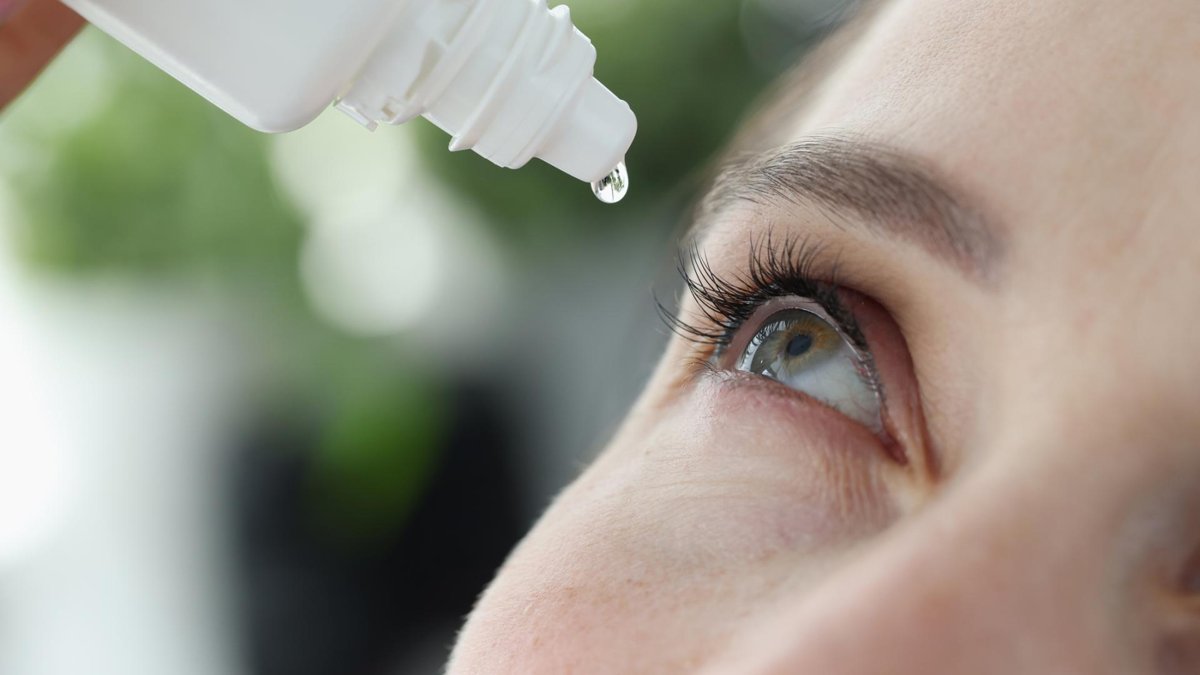
As we age, presbyopia—the gradual loss of the eye's ability to focus on near objects—becomes a common challenge, affecting over a billion people worldwide. For many, this means relying on reading glasses, but recent research suggests a promising alternative: eye drops that could potentially eliminate the need for them. In this article, we break down the latest studies on these innovative treatments, exploring how they work, their efficacy, and what the future holds for vision correction. Drawing from cutting-edge 2025 research, we'll examine options like pilocarpine-diclofenac combinations and FDA-approved drops such as Vuity and VIZZ.
Understanding Presbyopia: The Age-Related Vision Thief
Presbyopia typically begins in our 40s as the eye's lens loses flexibility, making it hard to focus on close-up tasks like reading or using a smartphone. This condition impacts nearly 90% of people over 45, leading to frustration and dependence on corrective lenses. Traditional solutions include reading glasses, bifocals, or surgery, but these aren't ideal for everyone—glasses can be inconvenient, and surgery carries risks. Enter pharmacological innovations: eye drops that target the root causes of presbyopia, offering a non-invasive, daily option to restore near vision without compromising distance sight.
How These Eye Drops Work: The Science Behind the Solution
Most presbyopia eye drops function as miotics, agents that constrict the pupil to create a "pinhole effect," enhancing depth of field and improving focus on near objects. For instance, pilocarpine, a key ingredient in many formulations, contracts the eye's ciliary muscle and narrows the pupil, mimicking the eye's natural focusing mechanism. When combined with diclofenac, an anti-inflammatory NSAID, it reduces potential irritation and inflammation, allowing for safer, more sustained use. Newer options like aceclidine-based drops (e.g., VIZZ) target the iris sphincter muscle selectively, avoiding effects on the ciliary body to prevent headaches or dim vision. These drops typically activate within 15 minutes and can last 6-10 hours per dose, providing all-day relief with just one or two applications.
The Groundbreaking 2025 ESCRS Study: Pilocarpine-Diclofenac Breakthrough

A pivotal retrospective study presented at the 43rd Congress of the European Society of Cataract and Refractive Surgeons (ESCRS) in Copenhagen on September 14, 2025, analyzed 766 patients treated with pilocarpine-diclofenac eye drops over two years. Led by Dr. Giovanna Benozzi from the Center for Advanced Research for Presbyopia in Buenos Aires, the research evaluated three concentrations: 1%, 2%, and 3% pilocarpine with a fixed diclofenac dose.
Results were impressive: In the 1% group (148 patients), 99% could read two or more extra lines on the Jaeger near-vision chart. For the 2% group (248 patients), 69% gained three or more lines, and in the 3% group (370 patients), 84% achieved the same improvement. Effects were rapid—within one hour—and sustained for up to two years with twice- or thrice-daily use. This real-world, single-center study marks the first systematic comparison of these concentrations, highlighting personalized dosing based on presbyopia severity.
FDA-Approved Options: Vuity and the New VIZZ Drops
Building on earlier research, Vuity (pilocarpine HCl 1.25%), approved by the FDA in 2021, has set the stage for presbyopia treatment. Phase 3 GEMINI trials involving 750 participants aged 40-55 showed users reading three or more extra lines on vision charts without losing distance acuity, with effects lasting up to 6 hours (extendable to 9 hours with a second dose).
In 2023, the FDA approved twice-daily dosing based on the Virgo trial. Fast-forward to 2025: VIZZ, an aceclidine-based drop from Lenz Therapeutics, received FDA approval in August, offering up to 10 hours of near-vision improvement without blurring distance vision or causing a "zoomed-in" effect.
Unlike pilocarpine, VIZZ spares the ciliary muscle, reducing side effects, and is set for broad U.S. availability in Q4 2025. These approvals signal a shift toward pharmacological management, with Vuity already demonstrating strong market performance and real-world efficacy for mild to moderate presbyopia.
Efficacy and Safety: What the Data Shows
Across studies, these drops consistently improve near visual acuity. The ESCRS research reported dose-dependent gains, with higher pilocarpine concentrations yielding better results for severe cases, and 99% of low-dose users achieving optimal vision. Vuity's trials confirmed statistically significant enhancements in low-light conditions, while VIZZ excels in providing extended, blur-free relief. Safety profiles are favorable: Common side effects include temporary dim vision, eye redness, irritation, and headaches, affecting >5% of users but resolving quickly.
No serious adverse events were noted in the two-year ESCRS follow-up, and systemic absorption is minimal. However, long-term data beyond two years is limited, and the retrospective nature of some studies calls for multi-center trials to confirm generalizability. Experts like ESCRS President-Elect Prof. Burkhard Dick emphasize the need for broader research to ensure widespread recommendation.
Limitations, Side Effects, and Who It's For
While promising, these drops aren't a cure-all. They work best for early to moderate presbyopia (ages 40-55) and may be less effective for advanced cases or those with cataracts.
Low-light performance can be reduced due to pupil constriction, and costs (around $79/month for Vuity, uninsured) may deter some. Not suitable for children under 18 or those with certain eye conditions. Side effects like headaches or irritation occur in a minority, but patients with dark irises may need higher doses due to melanin binding. Always consult an eye care professional for personalized advice, as these drops complement rather than replace glasses for everyone.
The Future of Presbyopia Treatment: Beyond Drops
The 2025 advancements, including the ESCRS study and VIZZ approval, herald a new era where eye drops could make reading glasses obsolete for millions.
Ongoing research explores combinations (e.g., miotics with lens softeners) and long-term quality-of-life impacts, with Dr. Benozzi planning further trials on physiological mechanisms. As pharmacological options expand, they offer freedom from glasses' inconvenience, potentially integrating with surgeries like LASIK for comprehensive care. With presbyopia affecting half of U.S. adults, these innovations could transform daily life, but rigorous, multi-year studies will be key to unlocking their full potential.
Conclusion: A Clearer Path to Better Vision
Eye drops like those studied in 2025 represent a revolutionary step in presbyopia management, providing safe, effective alternatives to reading glasses with minimal disruption. From the dose-dependent success of pilocarpine-diclofenac to the extended relief of VIZZ, research shows these treatments can restore near vision for hours at a time. While not without limitations, their benefits outweigh drawbacks for many, promising a glasses-free future.
If you're over 40 and struggling with close-up focus, discuss these options with your doctor— the end of reading glasses might be just a drop away.
NHPC enacts plan as Salal reservoir's capacity in Jammu & Kashmir shrinks 96% due to siltation
NHPC has launched a major desilting operation at the Salal Dam reservoir in Jammu and Kashmir, where silt buildup has reduced capacity by 96%, aiming
SBI aims to hike its green advances portfolio up to 10 pc by 2030
State Bank of India sets ambitious target to boost its green advances to 7.5-10% of total portfolio by 2030, supporting India's sustainable energy shi
After 14-hour surgery, Dipika Kakar confronts another medical challenge
Television actress Dipika Kakar faces a new liver cyst just months after her grueling 14-hour cancer surgery, prompting another medical procedure for
FMCG makers looks volume-based growth in FY’27 with EBITDA improvements as inflation softens
FMCG companies in India are shifting towards volume-driven growth in FY27, anticipating EBITDA improvements as softening inflation eases input costs a











